
Address:
- Hematology Division
Mail Stop 8125-0020-08, Washington University, 660 South Euclid Avenue, St. Louis, MO 63110 (mail)
Clinical Interests: Hemostasis
Research Interests: Gene therapy for blood protein deficiencies; Liver regeneration; Liver-specific function
Research: The focus of this lab is to use hepatic gene therapy to treat genetic deficiencies such as hemophilia or mucopolysaccharidosis VII (MPS VII). Hemophilia results in a lifelong bleeding diathesis due to a deficiency in any of several different coagulation factors. Continuous secretion of the deficient coagulation factor from the liver into the blood could result in the permanent correction of bleeding manifestations in these patients. MPS VII is due to a deficiency in the lysosomal enzyme beta-glucuronidase (GUSB) and results in bone and joint, cardiac, eye, and neurological abnormalities. For this disorder, the liver can secrete enzyme with a mannose-6-phosphate (M6P) tag that can be taken up by cells in the rest of the body via the M6P receptor. This laboratory has focused upon treating these genetic disorders with retroviral vectors, which can transduce up to 10% of hepatocytes in vivo when IV injection is performed in the setting of hepatocyte replication. The requirement of replication for efficient transduction of cells with this vector allows specificity for the liver to be achieved after systemic administration. Transfer into adults is achieved by inducing hepatocyte replication with the transient administration of hepatocyte growth factor, which is a growth factor that is selective for inducing hepatocyte replication in vivo after IV administration. Transfer of retroviral vector into neonates does not require any stimulus for hepatocyte replication, as the baseline level of replication is already high. Utilization of a strong liver-specific promoter that does not extinguish expression from a retroviral vector in vivo has allowed us to achieve stable and therapeutic levels of expression for over one year in rodents of Factor IX, which is deficient in one form of hemophilia. Similarly, this approach has resulted in high serum levels of GUSB with a M6P tag in mice with MPS VII, resulting in uptake in peripheral organs and amelioration of the disease.
Prior to using gene therapy to treat humans with genetic diseases, it will be necessary to demonstrate that these approaches are equally effective in larger animals. We have demonstrated that 9% of hepatocytes can be transduced when a retroviral vector is injected IV into neonatal dogs. When a retroviral vector expressing the canine GUSB cDNA was injected into neonatal MPS VII dogs, stable and high level expression of GUSB with a M6P tag was achieved for over 6 months. This resulted in a marked or complete improvement in the bone, cardiac, and ocular manifestations of the disease. Five out of 6 retroviral vector-treated animals could walk at 6 months of age, although no untreated mutants could walk at this time. This approach appears to be safe and effective at long-term correction of this disorder, although longer analysis will be necessary.
Future directions in the laboratory will include long-term evaluation of the efficacy and safety of this neonatal approach in large animals, and testing if it is equally effective in treating the bleeding manifestations of hemophilia B in dogs. We will also test the efficacy of hepatocyte growth factor-facilitated gene transfer in large animals, which would allow the application of retroviral vector-mediated gene transfer to older animals. In addition, a major focus will be to try to block immune responses that occur in the setting of gene therapy, as patients with large deletions or stop codons might produce antibodies or a cytotoxic T lymphocyte response to the therapeutic gene product.
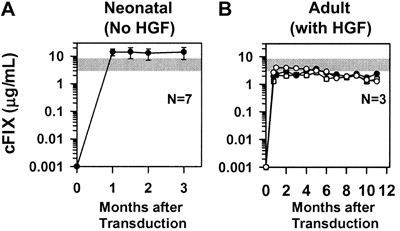
(A) Neonatal normal BALB/c mice were injected intravenously with a single dose of retroviral vector at 2 or 3 days after birth. Plasma factor IX levels were determined by immunoassay. The normal range is indicated by a gray bar.
(B) Adult BALB/c mice were injected intravenously with retroviral vector after administration of hepatocyte growth factor.
From: Xu L, Gao C, Sands MS, Cai SR, Nichols TC, Bellinger DA, Raymer RA, McCorquodale S, Ponder KP
Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B.
Blood 2003 May 15;101(10):3924-32
Biosketch
Education
- 1983: MD, Washington University, St Louis, MO
- 1979: BS in Biology, Stanford University, Stanford, CA
Post-Graduate Training
- 1990-1988: Postdoctoral Associate with Dr. Savio L.C. Woo, Department of Cell Biology, Baylor College of Medicine, Houston, TX
- 1988-1985: Postdoctoral Associate with Dr. Joan A. Steitz, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT
- 1985-1983: Residency in Internal Medicine, Parkland Hospital, Dallas, TX
Academic Positions
- 2019-present: Professor Emerita, Department of Medicine, Washington University School of Medicine, St. Louis, MO
- 2019-2007: Professor, Department of Medicine, Washington University School of Medicine, St. Louis, MO
- 2007-1998: Associate Professor, Department of Medicine, Washington University School of Medicine, St. Louis, MO
- 1998-1990: Assistant Professor, Departments of Medicine and Biochemistry & Molecular Biophysics, Washington University School of Medicine, St. Louis, MO
Board Certification
- Internal Medicine
Honors & Awards
- Phi Beta Kappa; Cum Laude (Stanford University)
- Antoinette Frances Dame Prize in Physiology and Biophysics
- George F. Gill Prize in Anatomy
- Kehar S. Chouke Prize in Anatomy
- Margaret G. Smith Award
- Medical Center Alumni Scholarship Fund Prize
- Merck Manual Award
- Upjohn Achievement Award for Research
- Alpha Omega Alpha
- American Medical Women’s Association Scholarship Achievement Award
- Lifetime Award, Sonia Skarlatos Public Service Award, American Society of Cell and Gene Therapy, May 2016
Peer-Review & Editorial Responsibilities
- 2018-2008: Editorial Board, Gene Therapy
- 2018-2005: Editorial Board, Human Gene Therapy
- 2018-2005: Editorial Board, Molecular Therapy,
Associate Editor, 2008-2005 - 2008-2003: Editorial Board, Blood
- 2010-2002: Reviewer, National Hemophilia Foundation Grants
- 20021998: Member, Medical Biochemistry Study Section
Professional Societies & Organizations
- American Society of Gene and Cell Therapy
Member, Advisory Council, 2018-2009 - National Hemophilia Foundation
Member, Medical and Scientific Advisory Council, 2012-2005 - American Society of Gene Therapy
Member, Education Committee, 2005-2001
Chair, Committee on Genetic Diseases, 2006-2003
Chair, Program Committee, 2007-2005
Member, Board of Directors, 2009-2006
Member, Advisory Council, 2018-2008 (Chair, 2013-2011) - American Society of Gene Therapy
Member, Board of Directors, 2009-2006 - American Society of Hematology
Selected Publications
- Measurement of Somatomedin-Related Peptides in Fetal, Neonatal, and Maternal Rat Serum by Insulin-Like Growth Factor (IGF) I Radioimmunoassay, IGF-II Radioreceptor Assay (RRA), and Multiplication- Stimulating Activity RRA After Acid-Ethanol Extraction
Daughaday WH, Parker KA, Borowsky S, Trivedi B, Kapadia M
Endocrinology 1982 Feb;110(2):575-81 - The Protease Specificity of Heparin Cofactor II. Inhibition of Thrombin Generated During Coagulation
Parker KA, Tollefsen DM
J Biol Chem 1985 Mar 25;260(6):3501-5 - Structural Analysis of the Human U3 Ribonucleoprotein Particle Reveal a Conserved Sequence Available for Base Pairing With Pre-rRNA
Parker KA, Steitz JA
Mol Cell Biol 1987 Aug;7(8):2899-913 - An in Vitro Interaction Between the Human U3 snRNP and 28S rRNA Sequences Near the Alpha-Sarcin Site
Parker KA, Bruzik JP, Steitz JA
Nucleic Acids Res 1988 Nov 25;16(22):10493-509 - Analysis of Pre-rRNAs in Heat-Shocked HeLa Cells Allows Identification of the Upstream Termination Site of Human Polymerase I Transcription
Parker KA, Bond U
Mol Cell Biol 1989 Jun;9(6):2500-12